Diseases – Quick Links
Here we provide information about Borreliosis (Lyme disease), the background of the disease, its signs and symptoms, diagnosis and treatment. You can also find specific answers to frequently asked questions in our FAQ section.
Follow the links below to go to specific sections, or go straight to the first section.
- Background
- Signs & Symptoms
- Early Localised Borreliosis
- Early Neuroborreliosis
- Early Disseminated Borreliosis
- Late Borreliosis
- Acrodermatitis Chronica Atrophicans (ACA)
- Arthritis
- Chronic Neurological Syndromes
- Diagnosis
- Testing
- Treatment
- The Jarisch-Herxheimer Reaction
- Post-Lyme Disease Syndrome
- Transmission
1. Background
Borreliosis [bore-El-ee-Oh-sis] (Lyme disease) is caused by a slow-growing spirochaetal bacterium of the Borrelia genus.
Borrelia Burgdorferi Spirochaete
The list of distinct species of borrelia spirochaetes continues to grow, with 48 named by the beginning of 2016. These have been divided into three groups with 21 species carried by hard bodied ticks placed in the genus of Borrelia burgdorferi sensu lato. Apart from a few species that feed only on lizards all others have been classified in the Relapsing Fever group which almost exclusively contains species carried by soft bodied ticks, with the notable exception of Borrelia miyamotoi carried by hard bodied ticks of the Ixodes species, and B. recurrentis carried by the human body louse.
The first species to be identified was discovered by Willy Burgdorfer, and named after him, in ticks associated with an outbreak of juvenile arthritis in the towns of Lyme and Old Lyme in Connecticut. The disease was named Lyme disease by Alan Steere who first investigated the cases. The disease was sometimes accompanied by a characteristic bull’s eye rash. A similar disease characterised by a skin rash had been recognised in Europe and described distributed in both the northern and southern hemispheres.
The Borrelia burgdorferi sensu lato group contains a number of species that are known to cause Lyme borreliosis (Lyme disease). The species that are confirmed to be pathogenic to humans continues to rise with 7 considered pathogenic,(B. afzelii, B. burgdorferi (sensu stricto), B. garinii, B lucitaniae, B spielmanii, B valaisiana). Some evidence exists for the pathogenicity of B bissettii and B bavariensis. Borrelia miyamotoi though classified in the relapsing fever group is pathogenic and produces symptoms typical of Lyme disease. Without doubt more borrelia species will be detected in the future and more identified as pathogenic causing Lyme like symptoms.
2. Signs & Symptoms
Borrelia spirochaetes can infect every organ of the body and the damage can manifest in many different ways. Since the bacteria enter the body at the site of an infected tick bite an early symptom is a rash. Other common symptoms include arthralgias, myalgias, fatigue, cognitive disfunction when the bacteria invades joint cartilage, muscles, nervous system and brain. A common characteristic of Lyme disease is that a single patient will frequently exhibit many of these symptoms and in a relapsing remitting manner.
3. Early Localised Borreliosis
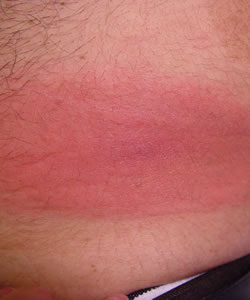
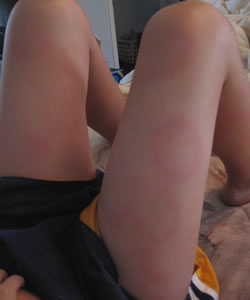
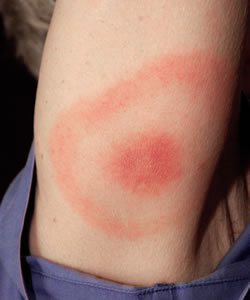
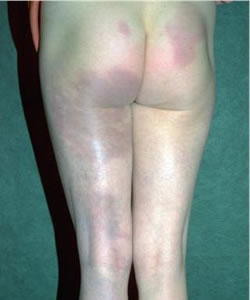
The first stages of disease can be a single, expanding Erythema Migrans (EM or “bull’s-eye”) rash, which may last for weeks. However, the rash may be absent or remain hidden under hair or in an inaccessible place. Epidemiological data from the Health Protection Agency over the last few years has demonstrated that fewer than 50% of laboratory-confirmed cases have reported a rash.
There can be a significant range of rashes beyond the classic, target-like EM, including multiple, flat, raised, or blistering rashes. The rash may also vary in colour from light pink to dark purple. Because of the variations misdiagnoses can occur and allergic reaction, ringworm, cellulitis and other more common skin ailments can be considered the cause. Rashes can also be missed if they are faint or if the patient’s skin is dark.
Recent evidence suggests that B. afzelii can result in more ring-like lesions while B. garinii can result in more irregular lesions, which tend to develop more rapidly.
Solid Lesion (source DermAtlas) Irregular Lesion (source DermAtlas)
Multiple Lesions (source DermAtlas) Target Lesion (source CDC)
Other early symptoms tend to be flu-like (mild and without the runny nose, cough and sore throat).
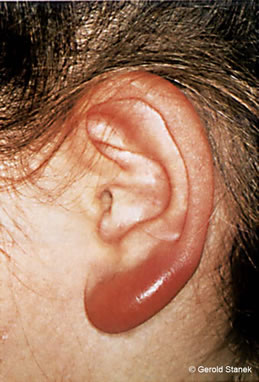
Borrelia lymphocytoma is an uncommon form of early disease. It is normally seen on the earlobe, nipple or scrotum. It appears as a dark, bluish-red, painless patch of skin.
Borrelial Lymphocytoma (©Gerald Stanek)

4. Early Neuroborreliosis
During this stage the Borrelia spreads into other tissues which can include the skin, nervous system, heart and musculoskeletal system. This can result in a wide variety of signs and symptoms which may present from a few weeks to over a year after the initial infection.
More severe symptoms can include multiple EM (often smaller than an initial lesion), joint and muscle pain and inflammation, radiculopathy (usually occurring months rather than weeks after infection) and carditis with conduction defects (usually partial heart block).
None of the presentations are unique to Borreliosis but combined with a history of tick exposure, or confirmed tick bite, are suggestive of the condition.
5. Early Disseminated Borreliosis
The presentation of early neuroborreliosis can include facial or other cranial nerve palsies (paralysis), aseptic (viral-like) meningitis or encephalopathy, Polyradiculitis and peripheral neuritis. Other manifestations such as transverse myelitis, cardiomyopathy, anterior and posterior uveitis, panophthalmitis, hepatitis, myositis and orchitis have been reported.
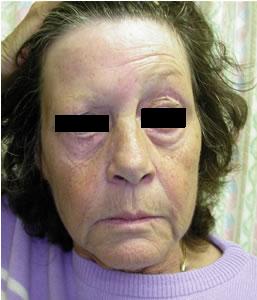
Facial Palsy (source BMJ)
6. Late Borreliosis
Late disease presents several years after the initial infection and may involve the skin (Acrodermatitis Chronica Atrophicans), the joints (arthritis) or chronic neurological syndromes.
7. Acrodermatitis Chronica Atrophicans
Acrodermatitis Chronica Atrophicans (ACA) is a dermatological manifestation of Borreliosis which can take a chronically progressive course and finally leads to a widespread atrophy of the skin. Involvement of the peripheral nervous system (predominantly sensory polyneuropathy) is frequently observed. The condition is due to the effects of continued active infection. Live spirochaetes have been isolated from skin biopsies as long as ten years after the patient was initially infected.
Acrodermatitis chronica atrophicans
©DermIS 1996 – 2012
Acrodermatitis chronica atrophicans
©DermIS 1996 – 2012
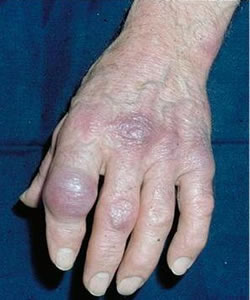
8. Arthritis
In the UK and most of Europe, Lyme arthritis is rare. It is more frequently seen in North America. It typically presents as a swelling of the large joints, most often the knee, but rarely causes erosion to the cartilage or bone. Direct infection of the joint has been implicated by culture of spirochaetes or DNA detection in joint fluid. Some patients present with inflammation of the tendons attached to the bone.
9. Chronic Neurological Syndromes
Direct infection of the nervous system can result in encephalopathy. This may present as memory loss, depression, sensory polyneuropathy or spastic paraparesis. Some manifestations of encephalopathy may resemble Chronic Fatigue Syndrome (CFS) or Fibromyalgia (FM). However, CFS and FM may be triggered by a preceding Bb infection (along with many other possible triggers) but evidence of ongoing, active infection may not be present.
10. Diagnosis
It is important for a patient’s history of possible tick exposure to be taken into account. Lyme borreliosis is endemic throughout the UK and Ireland, although some areas pose a higher risk than others. Public health England advises that any area that supports a hard tick population poses a risk. However, an assessment of tick exposure can be problematic as it is also recognised that ticks can be present in urban parks and gardens as well as rural areas. Bites may occur in places that are not considered to be risk areas. It has been highlighted by the World Health Organisation that, “In urban areas, nests of feral pigeons in the lofts of houses can result in invasions of soft ticks (such as the pigeon tick, Argas reflexus) into closely situated flats and apartments” and, “blackbirds in urban parks are carriers of infected ticks”. Other urban wildlife species, such as foxes and hedgehogs, can also carry and transport infected ticks.
A diagnosis can be problematic if the patient does not recall a tick bite. Over the last few years, epidemiological data from the Public Health England has demonstrated that fewer than 50% of laboratory-confirmed cases of Lyme borreliosis reported a tick bite. It is therefore important to take into consideration a patient’s recreational and occupational activities.
Diagnosis can also be problematic if a patient is infected with other tick-borne pathogens concurrently (co-infections) as this may alter the way in which Borreliosis presents, as well as the way in which it should be treated.
11. Testing
In the UK, laboratory tests for Borreliosis consist of a two-tier system which detects antibodies to Borreliae. The first of these is an Enzyme Linked Immunosorbent Assay (ELISA). This test superseded the Immunoflorescence Assay (IFA) and is the most commonly used serodiagnostic screening method for Lyme borreliosis.
If the ELISA is positive or equivocal, the sample is then tested with an Immunoblot, also known as a Western blot. Western blot, ELISA and PCR (polymerase chain reaction) can be performed on blood or cerebrospinal fluid (CSF), which is obtained via a lumbar puncture. However, antigen capture in CSF can be extremely elusive; reportedly CSF yields positive results in only 10-30% of patients cultured. Therefore, the diagnosis of neurologic Borrelial infection should not be excluded solely on the basis of a negative CSF antibody analysis.
The detection of Borrelia DNA using PCR can also be performed. This type of molecular test can be useful on joint fluids in cases of suspected Lyme arthritis, and on biopsies from suspected skin infection. It isn’t normally used for blood samples as Borreliae are rarely present in the bloodsteam after the early stage of infection. PCR isn’t favoured for CSF.
All methods of testing have their limitations and can produce both false-positive and false-negative reactions. Antibodies may not be present for the first few weeks after infection so a negative test does not exclude infection. A second sample taken 2-4 weeks later may then go on to show seroconversion. In late stage disease, patients can be seronegative although this is considered a rare phenomenon.
False-positive results can occur if the patient has antibodies to Bb without having a current infection (e.g. people who are occupationally exposed, such as foresters, or people who are recreationally exposed to tick bites).
The significance of any result, negative or positive, should be interpreted carefully by clinicians in the overall context of a patient’s clinical findings and tick-exposure history. Other conditions such as glandular fever, syphilis, or certain neurological illness can also trigger a false-positive reaction.
Other techniques for testing are in development but their clinical usefulness are the subject of debate and have yet to be adequately established.
12. Treatment
Treatment has become a controversial issue over recent years. Although most doctors would agree that treatment of a Borrelial infection (in its early, localised phase) is clinically successful in most cases, there is far less agreement regarding the treatment of neuroborreliosis and disseminated infection.
In most cases treatment involves a course of oral antibiotics but in cases of neuroborreliosis or cardiac complications, intravenous antibiotics may be required.
13. The Jarisch-Herxheimer Reaction
The Jarisch-Herxheimer reaction (also referred to as a J-H reaction, Jarisch-Herxheimer, or a herx) can occur during the treatment of Borreliosis. It is believed to result from large quantities of endotoxin-like substances (toxic structural components of bacteria) being released into the body as bacteria are lysed (killed) by the antibiotic therapy. It is thought that the release of endotoxins occurs faster than the body can remove them through the natural detoxification process performed by the liver and kidneys.
The J-H reaction is most commonly associated with the treatment of syphilis (another spirochaetal infection), and was named after two dermatologists (Adolf Jarisch and Karl Herxheimer) who first observed the reaction in syphilis patients during treatment. The reaction also occurs in the treatment of tick-borne Relapsing fever, Q-fever and certain other diseases.
The J-H reaction typically manifests between 1 and 12 hours after the initiation of antibiotic therapy. Symptoms can include fever (generally low grade), chills, headache, myalgias, rigors, hyperventilation, tachycardia, hypertension followed by hypotension (due to vasodilation and declining peripheral resistance), and an exacerbation of cutaneous lesions (which can be mistaken for an allergic reaction to treatment). Careful management (supportive therapy and the use of certain medications such as Diphenhydramine hydrochloride) can help avoid premature cessation of antibiotic treatment.
There is some disagreement regarding the duration of the J-H reaction in the treatment of Borreliosis, with reports varying from a few hours to repeated reactions during the course of treatment.
14. Post-Lyme Disease Syndrome
Some patients with Lyme disease still experience symptoms (such as fatigue, soreness and memory or concentration loss) after their treatment has finished. They are sometimes diagnosed as having chronic Lyme disease or post-Lyme Disease Syndrome. What causes these symptoms has become the subject of controversy. One side of the argument is that immunologic mechanisms are triggered by the initial infection, leading to long-term symptoms even after treatment (Post-Lyme Disease Syndrome). The other is that the stealth nature of the bacterium may lead to treatment failure and therefore some doctors believe that longer, more aggressive treatment is required. Both treatment failure and Post-Lyme Disease Syndrome have been poorly defined and currently no definitive test exists to determine whether an infection has completely resolved.
New research has established that patients diagnosed with Post -Lyme Disease Syndrome have antibodies that suggest they carried the infection for an unusually long time. Researchers discovered that antibodies in these patients recognise changes in the bacteria’s outer surface protein which leads to the patients having a greater variety of antibodies than those whose infection cleared up quickly.
This finding suggests that patients with chronic symptoms have experienced a prolonged infection, caused by microbes that have evaded the immune system. The researchers suggest this could mean that the patients naturally have a different antibody response to the infection than most people, or that they weren’t treated properly, or that they were re-infected and the second infection was never treated. The study is the first of its kind which demonstrates immunologic difference between someone who resolves their infection and someone who develops post-Lyme Disease Syndrome. The presence of varied antibodies hints that the chronic symptoms could be caused by an ongoing inflammatory response caused by antibodies mistakenly reacting to the body’s own proteins.
Reference:
15. Transmission
Other than being a tick-borne disease, Borreliosis may be passed from an infected mother to her foetus. It may also be passed through transfusion of infected blood. Borrelia bacteria can survive for up to 48 days at 4 degrees centigrade in human blood processed for transfusion.